Aluminium production
Explore the history and process of aluminium production, from bauxite to metallic aluminium. Learn about the revolutionary breakthroughs that led to its widespread use in transportation and electrification.
Industrial metal
The revolutionary technology for the electrolytic reduction of aluminum oxide (Al2O3), dissolved in molten cryolite, was opened in 1886 year. This event coincided with three other revolutionary breakthroughs in technology [1]:
- The first vehicles powered by internal combustion engines. Aluminum value, as the structural material, has increased dramatically.
- Electrification required huge amounts of lightweight electrically conductive material to transmit electricity over long distances and build towers to support electrical cables.
- New industry – aircraft manufacturing. Because aluminum produced aircraft frame, engines and other parts and units.
Primary aluminum
The process
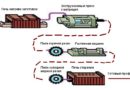
The modern process of production of primary aluminium ingots is shown in Figure 1. Bayer method is used to convert bauxite to alumina, which in the aluminum industry is called alumina (upper part of Figure 1). The alumina is then reduced to metallic aluminium (lower part of Figure 1).
 Figure 1 – Primary aluminum production scheme:
Figure 1 – Primary aluminum production scheme:
from original bauxite to aluminium products [2]
- Alumina from bauxites.
- Alumina is dissolved in a bath of cryolite and fluoride salts.
- Through a bath is passed electric current to divide by electrolysis of aluminum oxide on oxygen and aluminium.
- Oxygen reacts with the graphite anodes. Molten aluminum collects at the bottom of the electrolysis unit (Figures 2 and 3).
- Liquid aluminum periodically pumped through siphon or special vacuum furnace to transmit to casting units.

Figure 2 – Alumina production. Bayer method [5]
Figure 3 – Primary aluminium smelting [3]
Figure 4 – The cross section of an electrolytic cell [5]
Inevitable impurities
Smelted main impurities of primary aluminum are iron and silicon, however, zinc, gallium, titanium and vanadium is usually always present in a different number of go. The purity of aluminum is estimated the maximum allowable amount of impurities. for instance, aluminum 99,70 % It contains not more than 0,30 % impurities.
Aluminum refining
To obtain a higher degree of purity aluminum using special technology. The purity 99,99 % is achieved by zone melting or processing of liquid aluminum using the Hoopes method (Figure 5).
Figure 5 – Furnace for refining aluminum according to the Hoops method [3]
The lower layer serves as an anode,. It consists of a refined (purified) aluminum alloy with copper. Copper is introduced in order, to increase the density of the lower layer. Middle layer – This molten electrolyte. Its density is lower, than the density of the anode and the alloy above, than density already refined aluminum, which the “floats” electrolyte top.
Purification of aluminum is due to dissolution of impurities on the anode as a result of electrochemical reactions.
Purification by zone melting aluminum
The principle of zone melting is repeated passes along the zone melting of aluminum ingot. impurities, which lower the melting point of aluminum, They accumulate in the melting zone and gradually move towards the end of the ingot. These contaminants include, for example, lead, beryllium, calcium, iron, cobalt, nickel, magnesium,, copper, silicon, zinc. impurities, which increase the melting point, concentrated at the beginning of the ingot. These contaminants include, for example, chromium, titanium, Molybdenum, vanadium. Manganese does not change the melting point and therefore does not move under the influence of the melting zone. Zone melting reach purity alumina 99,9999 % [2].
Secondary aluminium production
Source for secondary aluminium is aluminum scrap and aluminum waste in all forms and types of products, as well as slag and other waste aluminum foundries. Primary and secondary aluminum production are closely linked. Many aluminum alloys, wrought and cast alloys, suggest the presence of various impurities, which may be present in the aluminum scrap and aluminum waste processing.
Handbook of aluminium recycling /Ch. Schmitz – 2014
Aluminium recycling /M. Schlesinger – 2017
Aluminum alloys
Aluminum alloying
Pure aluminum has a very low strength and its use as a structural material is very limited.
When aluminum is added to the other elements – alloying elements – it increases its strength due to different hardening mechanisms.
Aluminium alloys, basically, possibly doped with a majority of the metallic elements. However, only some of them have a sufficient solubility in the solid state, to be the major alloying elements.
The most important alloying elements of aluminum are:
- copper;
- manganese;
- magnesium;
- silicon and
- zinc.
At the same time, a considerable number of other elements have a marked effect on improving the properties of aluminum alloys. They are added in small quantities. These elements include chromium, the same manganese and zirconium, which are used mainly for the control of grain structure.
The maximum solubility of alloying elements in aluminum are usually, but not always, It is achieved at the eutectic temperature. The solubility of alloying elements in solid aluminum decreases with decreasing temperature. This change in solubility in solid aluminum is the basis for strengthening aluminum alloys due to the aging mechanism.
Iron in aluminum
All industrial alloys contain about 0,1 to 0,4 % iron (by weight). Typically, the iron in the aluminum alloy is considered an impurity. Its content depends on the initial ore and electrolysis technology when smelting. Sometimes iron intentionally added to impart special properties of the material, for example, to 1 % in alloys for the manufacture of aluminum foil.
Additives in aluminum
In combination with one or more basic alloying elements, additional elements are often used:
- bismuth,
- brown,
- chromium,
- lead,
- titanium and
- zircon.
These elements are typically used in small amounts, usually, to 0,1 %. However, in some aluminum alloys boron content, lead and chromium can reach 0,5 %. With these alloys produced small corrections necessary properties for specific conditions, such as , good fluidity at casting, high quality machining, heat endurance, corrosion resistance, high strength.
Categories of aluminum alloys
It is convenient to divide aluminum alloys into two main categories:
- casting alloys and
- wrought alloys.
6xxx alloys
The most popular in global production aluminum profiles are 6xxx series alloys. These are aluminum alloys alloyed with magnesium and silicon, each containing about one percent. The European standard EN 573-3 It consists of approximately 30 pcs. Of these thirty alloys, aluminum alloys are the most widely used:
Of these five alloys in the world is made more 90 % all pressed aluminum profiles.
Aluminum products
Types of products
Aluminum and its alloys can be cast or molded into finished products and semi-finished products in almost any of the known processes, applied to metal. According to their shape of the product is divided into standard and “according to plan”.
The first ones include:
- sheets,
- plate,
- foil,
- rods,
- wire,
- pipe and
- standard profiles.
Product “customer drawings”, «engineered products», developed for some special application:
- extruded profiles,
- forgings,
- castings.
Aluminum extrusion
Extrusion of aluminum and its alloys is a process of plastic deformation, wherein the preform, usually part of a round ingot, extruded through one or more holes of the matrix – special pressing tool. To do this, use the special equipment – extrusion presses, usually, hydraulic, which provide force from 500 to 4000 tonnes, and sometimes more.
Details of Aluminum Extrusion:
Aluminum Extrusion Technology / P. Saha
Extrusion of Aluminium Alloys / T. Sheppard
Aluminum castings
Aluminum castings are usually produced by following methods:
- injection molding;
- casting into permanent molds;
- sand casting;
- casting in plaster molds;
- a meltable casting mold.
Aluminum forgings
Aluminum forgings produced creation plastic flow of metal by the application of kinetic therein, mechanical or hydraulic forces in the open or closed matrix. forgings, performed manually, have simple geometric shapes – rectangles, cylinders, drives. More complex forms of hammer in closed molds
Sources:
- Corrosion of Aluminum and Aluminum Alloys – ASM Speciality Handbook /ed. J.R. Davis, 1999
- Aluminum Electrical Conductor Manual – Aluminum Association, 1989.
- Handbook of Aluminum, Volume 1 – Physical Metallurgy and Processes / ed. George E. Totten, D. Scott MacKenzie, 2003.
- Материалы Алюминиевой ассоциации Германии
- TALAT 1101