The melting point of aluminium
Unlock the secrets of aluminium melting points. Dive into the science behind the variation in melting temperatures based on aluminum purity.
The melting point of pure aluminium
- The melting temperature of ultra-pure aluminum 99,996 % is 660,37 ° C.
- The melting temperature of 99,5 % aluminium is 657 ° C.
- The melting temperature 99,0 % aluminium is 643 ° C.
The melting point of metals
Metals and non-metals
Any piece of metal, for example, aluminum, millions of individual crystals, called grains. Each grain has a unique orientation of the crystal lattice, but together grain oriented within this piece of randomly. Such a structure is called a polycrystalline.
amorphous materials, for example, glass, different from crystalline materials, for example, aluminum, for two important differences, which are related to each other:
- absence of long-range order of the molecular structure
- differences in the nature of melting and thermal expansion.
Molecular structure difference a metal and a non-metal can be seen in Figure 1:
- On the left is shown tightly packed and ordered crystalline structure of a metal
- An amorphous material shown on the right: less dense structure and random arrangement of atoms.
 Figure 1 – Illustration of difference in structure between:
Figure 1 – Illustration of difference in structure between:
(a) crystalline and (b) non-crystalline materials.
The crystal structure is regular, repeating, and denser,
whereas the non-crystalline structure is more loosely packed and random [3]
Melting of a metal
This difference in the structure is manifested in melting metals, including, melting of aluminum of various purities and its alloys. Less densely packed atoms give an increase in volume (decrease in density) compared to the same metal in the solid crystalline state.
In pure metals, this volume change occurs very rapidly and at a constant temperature. This temperature is the melting point of a metal. The melting point gives the gap between the sloping lines on either side of the melting point. Both these oblique lines characterize the thermal expansion of the metal, which is usually a variety of liquid and solid state.  Fig. 2 – Characteristic change in volume for a pure metal (a crystalline structure),
Fig. 2 – Characteristic change in volume for a pure metal (a crystalline structure),
compared to same volumetric changes in glass (noncrystalline structure) [3]
The heat of fusion
With this dramatic increase in the volume of metal at the transition from the solid to the liquid state due a certain amount of heat, which is called the latent heat of fusion. This heat causes the atoms lose an ordered and dense crystal structure. This process is reversible, He works in both directions – and upon heating, and cooling.
The equilibrium melting temperature
As shown above, pure crystalline substance, for example, pure metals, have a characteristic melting temperature, often referred to as “melting point”. At this temperature, it is a pure crystalline solid is melted and becomes a liquid. The transition between the solid and liquid state for small specimens of pure metals is so small, that can be measured to an accuracy 0,1 oC.
Liquids have a characteristic temperature, where they are converted into solid. This temperature is called the solidification temperature or solidification point. In theory – under equilibrium conditions – the equilibrium solid melting temperature is the same, and that the equilibrium temperature of solidification. In practice, small differences can be observed between these values (Figure 3).
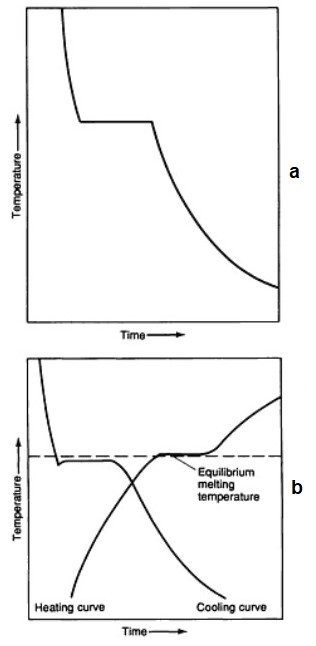 Figure 3 – (a) Ideal freezing curve of a pure metal;
Figure 3 – (a) Ideal freezing curve of a pure metal;
(b) Real freezing and heating curves of a pure metal [4]
Liquidus and solidus
- Temperature beginning melting is called the solidus temperature (or solidus point)
- Temperature end of melting – liquidus temperature (or liquidus point).
“Solidus” means, understandably, solid, and “liquidus” – liquid: at solidus temperature the entire alloy more solid, and at liquidus temperature – the whole already liquid.
When this alloy solidifies from a liquid state, the temperature of the onset of crystallization (solidification) will be the same liquidus temperature, a closure crystallization – the same solidus temperature. When temperature alloy between its solidus and liquidus temperatures it is in semi-solid semi-, mushy state.
Melting range of an aluminium alloy
The influence of alloying elements and impurities
- Adding other elements to aluminium, including alloying, lowers its the melting points, more precisely – the start melting temperature.
- So, some casting aluminum alloys with a high content of silicon and magnesium melting start temperature is reduced to almost 500 ° C.
- The term “melting point” is applied to pure metals. Alloys do not have a specific melting point.
- The process of full melting of aluminium alloys occurs in a certain temperature range.
 Figure 4 – Changes in volume per unit weight (1/density) as a function of temperature
Figure 4 – Changes in volume per unit weight (1/density) as a function of temperature
for a hypothetical pure metal, alloy, and glass;
all exhibiting similar thermal expansions and melting characteristics [3]
Melting ranges of aluminium alloys
Table 1 shows the solidus and liquidus temperature of some commercial wrought alloys. It must be borne in mind, that the concepts of the liquidus and solidus temperatures are defined for equilibrium reactions in the liquid phase and a solid back, ie at infinite duration processes. In practice, it is necessary to make adjustments based on the rate of heating or cooling.
Table 1 – The points of liquidus temperature and solidus temperature of some aluminium alloys [1]
Aluminuim solidification
Pure aluminium
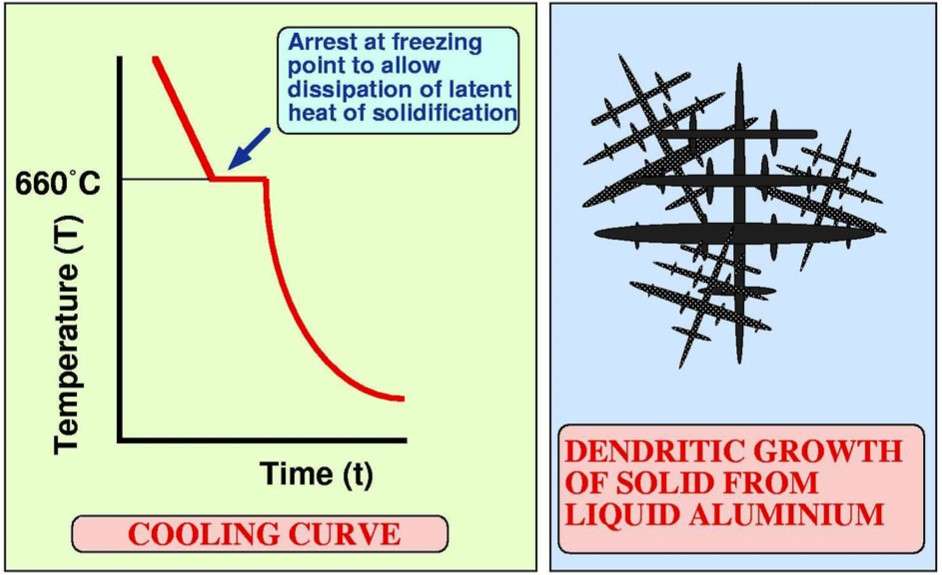
Pure metals, including, pure aluminum, have a clear melting temperature – a melting point. Solidification or “freezing” pure aluminum also occurs at a constant temperature. When pure molten aluminum is cooled, its temperature falls to freezing point and remains at that temperature, until all of it (liquid aluminum) hardens. In figures 5 и 6 typical cooling curves of pure metal with its transition from liquid to solid are shown.
 Figure 5 – Cooling curve for a pure metal during casting [3]
Figure 5 – Cooling curve for a pure metal during casting [3]
An aluminium alloy
During solidification of an aluminum alloy, which consists dissolved an alloying elements, shows, that the beginning of the solidification occurs at a temperature, and the ending is at a different temperature (figure 7). 
Figure 7 – Cooling curve for an aluminium alloy casting (adapted from [3])
The melting points of various pure metals
The melting point of some other pure metals is (degrees Celsius) [1]:
- mercury: minus 39
- lithium: 181
- lead: 232
- lead: 328
- zinc: 420
- magnesium: 650
- copper: 1085
- nickel: 1455
- iron: 1538
- titanium: 1670
Sources:
1. Aluminum and Aluminum Alloys, ASM International, 1993
2. Handbook of Aluminum: Vol. 1, ed. G. E. Totten, D. S. MacKenzie, 2003
3. Groover, Mikell P. Fundamentals of modern manufacturing: materials, processes and systems, 4th ed. – JOHN WILEY & SONS, 2010
4. Introduction to Alloy Phase Diagrams – ASM International, 1992
5. TALAT 1205


