How the Surface of Aluminium Determines Its Corrosion Resistance
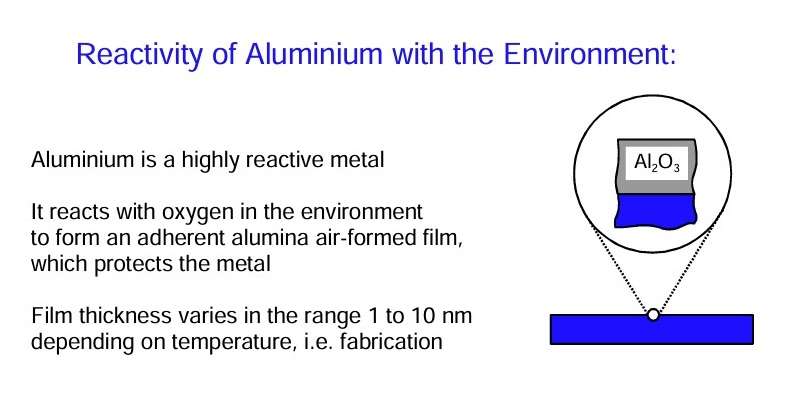
The effective use of aluminium is sensitive to its potential reactivity under various environmental circumstances. Fortunately, exposure of aluminium in many circumstances leads to the rapid production of an air-formed film of alumina.
- At ambient temperatures, the film is amorphous.
- For formation conditions at and above 500 ⁰C, both amorphous and crystalline aluminas are present.
- The amorphous film developed at room temperature have limiting thickness of about 2,5 nm, thereby stifling further oxidation of the substrate.
Figure 1
Video: How the Surface of Aluminium Determines Its Corrosion Resistance
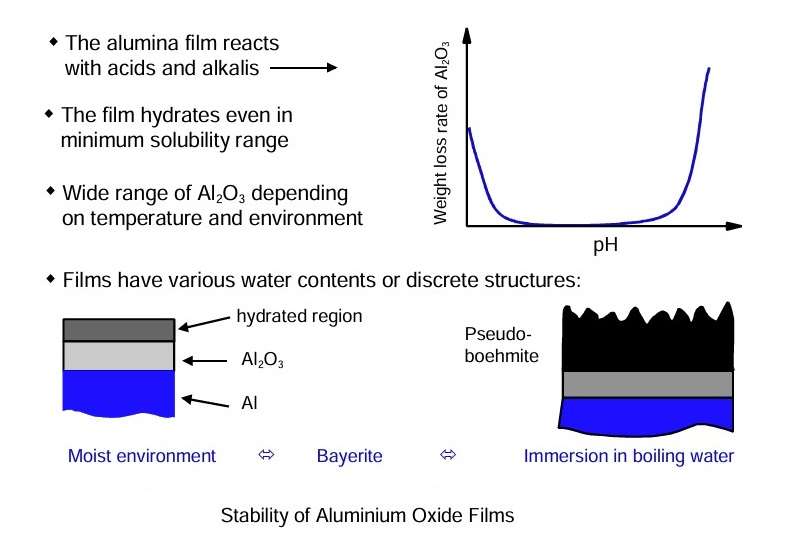
Depending on the particular circumstances, the established film will react further with the environment; thus, hydration of the outer surface of the alumina film can proceed to develop multi-layered films.
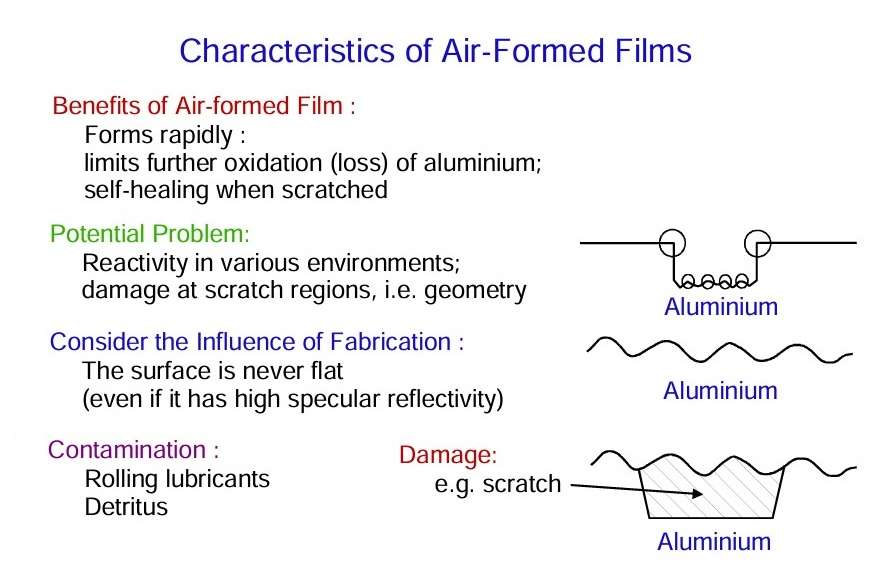
The high affinity of aluminium for oxygen, resulting in development of alumina films. It also ensures that similar films will develop in damaged or scratched regions of the substrate. The figure shows some characteristics of air-formed oxide films.
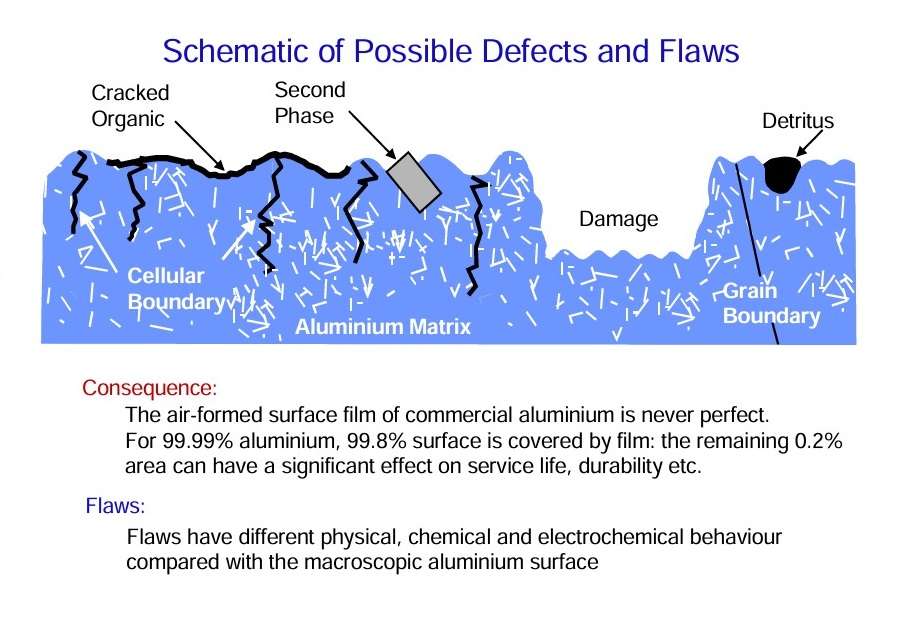
Defects of various kinds, generally called flaws, are present in the air-formed film on the aluminium surface. The total area occupied by such flaws is low, but processes proceeding at them largely determine the effective behaviour of aluminium.
What is a piece of aluminum made of:
- Aluminium has a close packed, face centred cubic arrangement of atoms comprising the unit cell.
- In a given grain, the unit cells are arranged in a systematic manner to provide the macroscopic material.
- For the bulk material, many different grains and their grain boundaries intersect the material surface. Such grains develop during casting, deforming and subsequent heat treatment.
- The material comprises grains of aluminium of various orientations separated by their grain boundaries.
- Clearly above the grains, the air-formed film is present, providing a barrier between the outside environment and the aluminium substrate.
- The macroscopic surface is relatively rough with undulations resulting from the particular fabrication process.
- Lubricating fluids employed in fabrication processes result in organic contaminations at the surface.
- Impurities rolled into the surface provide further regions of locally altered chemistry.
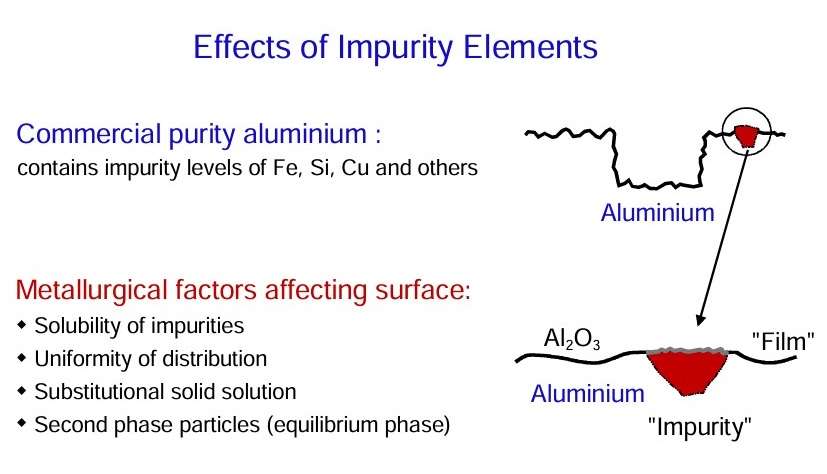
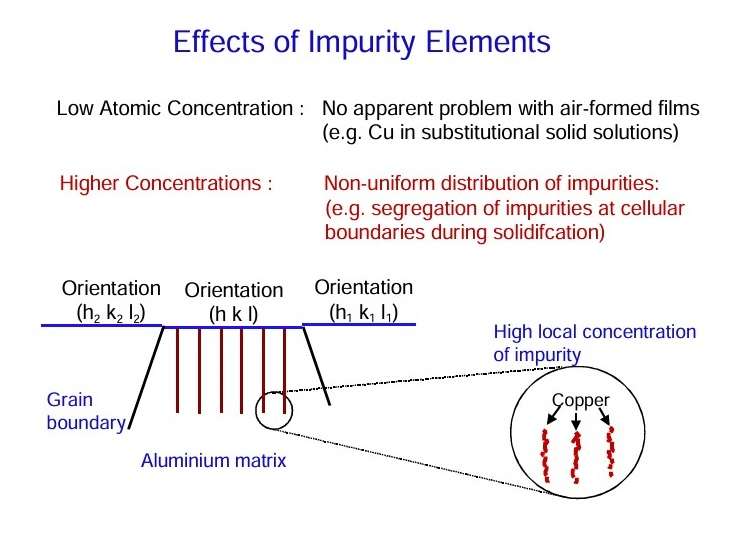
Several effects can arise from alloying of aluminium:
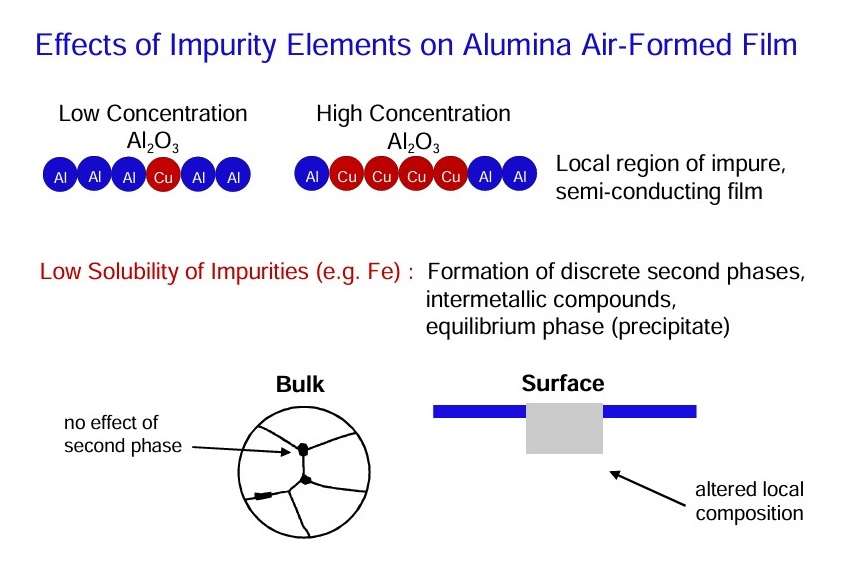
- Alloying additions may be present in substitutional solid solution in the aluminium matrix. However, once the primary solid solubility is exceeded, second phase or intermetallic constituents will develop.
- For the age-hardening alloys, comparatively fine intermediate phases also precipitate.
- The common impurity elements in alloyed aluminium are copper, silicon and iron.
- Iron has a very low solid solubility in aluminium. Thus, in commercial purity materials, iron is present as distinct second phase material of relatively coarse nature.
The surface nature of an aluminium alloy is far more complex than the relatively simple pure aluminium. Various features need to be considered are listed in Figure 7.
For a pure material, in single crystal form, the distribution of high energy or potentially active sites on the surface is relatively clear. Thus, in corrosion or metal dissolution, loss of metal ions occurs preferentially from kink sites at the surface or ledge sites as opposed to the lower energy terrace sites. However, for aluminium alloys, supporting their air formed films, any additional features arising influence particularly the activity of local regions at the surface.
Basically, сorrosion of aluminium is the process by which aluminum returns to the its inorganic compound form.
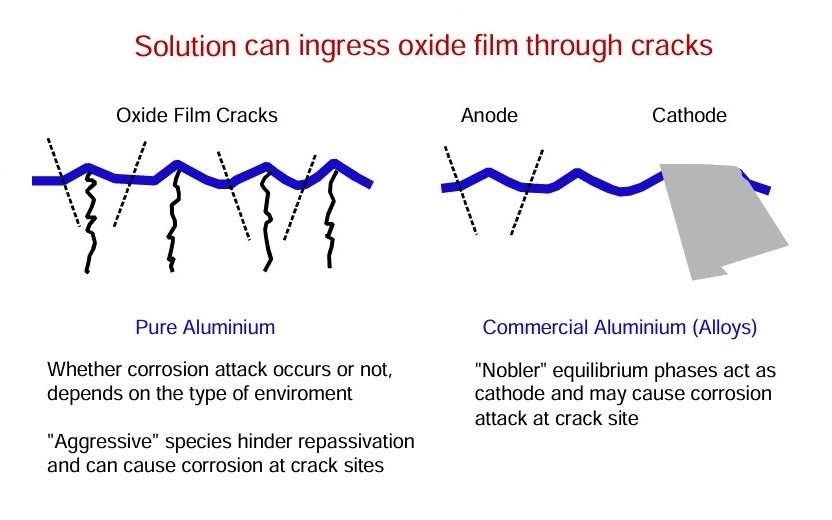
In low or high pH environments which are reactive to the alumina film, the metal will corrode in a general or uniform manner at a relatively slow rate.
For alloys, local heterogeneities in the material morphology and composition give rise to particular forms of corrosion, for example, intergranular corrosion and exfoliation corrosion, where dissolution follows to the grain boundaries. In situations where so-called aggressive anions are present, i.e. chloride, localized corrosion of the passive metal proceeds, initiating at sites where the alumina film is breached and where healing is hindered.
The source:
TALAT Lecture 5101 – Surface Characteristics of Aluminium and Aluminium Alloys / G.E.Thompson – European Aluminium Association – 1994