Protection of structural aluminium from corrosion
Discover the science behind structural aluminium corrosion and how to protect it. Learn about galvanic, pitting, and crevice corrosion and how to prevent it.
The natural oxide coating
Natural aluminium surface, that occurs during the manufacturing of aluminium products, for example, pressing, rolling or casting, It has a high resistance to corrosion in most types of environmental. This is because, that fresh aluminium surface spontaneously and instantaneously forms a thin, but very effective oxide layer, which prevents further oxidation of the metal.
A natural oxide layer is the main reason of good resistance to corrosion of aluminium. This coating is stable in acidic environments – with pH – from 4 to 9. However, in more difficult conditions, aluminium needs to be protected from corrosion.
Three main types of structural aluminium corrosion
The most common types of structural aluminium corrosion are:
- galvanic (contact) corrosion;
- pitting corrosion;
- crevice corrosion.
Stress aluminium corrosion, which leads to the formation of cracks, is a specific kind of corrosion. It happens mostly in high-strength aluminum alloys, for example, alloys AlZnMg, when subjected to prolonged tensile stress in the presence of the corrosive environment. This type of corrosion do not usually occur in structural alloys of the 6xxx series.
Galvanic corrosion of aluminium
Galvanic corrosion can occur when, When two different metals are in direct contact and an electrolyte bridge is formed between them. Less noble metal in this combination becomes the anode and corrodes. More noble metal becomes the cathode and is protected from corrosion by.
In most combinations with other metals, aluminum is less noble metal. Therefore, aluminum is exposed to a higher risk of galvanic corrosion, than other building materials. However, this risk is less, what is generally considered.
Prerequisites: contact and moisture
Galvanic corrosion of the aluminum occurs only, when at the same time:
- there is contact with a more noble metal (or other electrical conductor with a higher chemical potential, than aluminum, for example, graphite;
- between the two metals is an electrolyte with good conductivity, most often, Water with dissolved salts.
Galvanic corrosion occurs in a dry air atmosphere, for example, in normal premises. There is not much risk of galvanic corrosion and clean rural atmosphere. At the same time, the risk of galvanic corrosion must always be taken into account in atmospheres with a high chloride content, for example, in areas near seas and oceans.
Aluminum and galvanized steel
There can be problems with galvanic corrosion in aluminum and galvanized steel paired with. The zinc coating is first galvanized steel to protect aluminum from corrosion. However, this protection is reduced, when the surface starts to become naked as zinc spending. Hot-dip galvanized steel gives greater thickness of the zinc coating, than electrochemical galvanizing and provides longer-term protection of aluminum. Therefore, in an aggressive atmosphere in contact with the aluminum used only galvanized hot dip galvanized steel.
Electrical isolation
There, where different metals are applied in contact, galvanic corrosion can be avoided by the electrical insulation of one metal on another. An example of such solutions for the bolted connection between the aluminum and the steel sheet is shown in Figure 1. Between the bolt head and the aluminum surface may electrolyte, but electrically insulating washer will not allow the possibility of galvanic electrical current to flow and corrosion will not occur. On the other hand in contact aluminum and the steel sheet is no possibility of moisture, electrolyte is not formed and the corrosion does not occur.
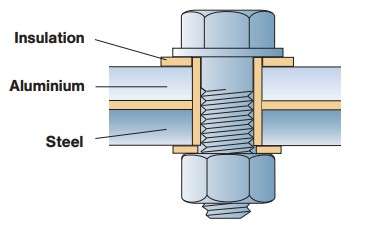 Figure 1 – Electrical isolation aluminium from steel
Figure 1 – Electrical isolation aluminium from steel
Electrical insulation
In large structures, there, wherein application of electrical insulation is difficult, apply alternative solution – preventing electrolytic bridge between the two metals. Painting of the surface – this is one of the ways to do it. In most cases, the best one is a coloring cathode surface, that is, a noble metal.
Cathodic protection
Cathodic corrosion prevention can be accomplished in two ways. Most often – setting the anode of the less noble metal in direct contact with aluminum metal. This less noble metal “sacrificing” a, ie corrodes instead of aluminum. Therefore, it is called a sacrificial anode.
To such a sacrificial anode worked, it must be in liquid contact with the aluminum surface to be protected. To protect aluminum as sacrificial anodes used most often zinc and magnesium. EXAMPLE cathodic protection is shown in Figure 2.
Another way of obtaining cathodic protection is connecting the aluminium object to the negative pole of the rectifier.
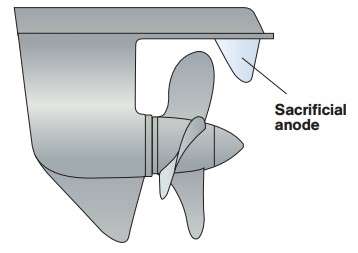 Figure 2 – Cathodic protection of aluminium screw vessel
Figure 2 – Cathodic protection of aluminium screw vessel
Pitting aluminium corrosion
Pitting alumini corosion is the most common type of corrosion. It also only happens in the presence of an electrolyte (water or moisture), which contains dissolved salts, usually chlorides.
This corrosion is usually looks like a very small pits, which outdoors reach a maximum depth of a small part of the thickness of the metal. The depth of these holes may be greater in water and soil.
Preventing pitting corosion
Pitting corrosion is mainly a matter of aesthetic, because, in practical terms,, never reduces the strength of aluminum products.
Manifestation of pitting corrosion, of course, It is more serious on aluminum with a natural surface, that is, the surface without any protective treatment. Protective surface treatment of aluminum (anodizing, painting or other coating methods) successfully protects it from pitting corrosion.
To prevent pitting corrosion, cathodic protection is also used Figure (Figure 2).
Construction of drainage
It is important to design the aluminum profiles and other aluminum products so, that they were able to drain rain and rapid drying surface. profiles, which may be exposed to moisture, You should not have corners or pockets, in which water accumulates. each profile, in which water can collect, must have drain holes (Figure 3).
Effective drainage (figure 4) and ventilation of “wet” aluminum profiles significantly reduces the risk of pitting corrosion on them.
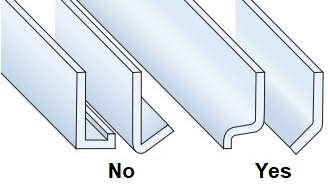 Figure 3 – To avoid pockets in which water can collect [1]
Figure 3 – To avoid pockets in which water can collect [1]
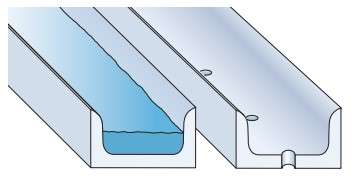 Figure 4 – Drainage holes in aluminium profile [1]
Figure 4 – Drainage holes in aluminium profile [1]
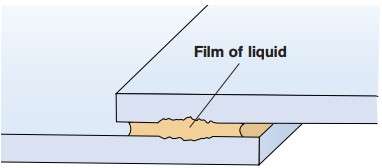
Crevice corrosion of aluminium
Crevice corrosion may occur in narrow, liquid-filled crevices. The occurrence of such corrosion in aluminum profiles unlikely. However, significant crevice corrosion may occur in a marine atmosphere or on the outer surface of the vehicle body. During transportation and storage of aluminum profiles can sometimes collect water in the gaps between adjacent aluminum surfaces, which causes surface corrosion in the form of “water spots” (Figure 4).
The source of this water is rain or condensation. This water on the capillary mechanism is literally sucked into the space between the two metal surfaces. Condensation may occur if, when the cold material is placed in a warm room. The difference between night and daytime temperatures can also cause condensation, when aluminum is stored outside under dense awning, which prevents ventilation.
Prevent Crevice Corrosion
On the mating surface applied adhesives or double-sided tape. This prevents ingress of water into the gap between them and prevents occurrence of crevice corrosion.
In some cases, instead of the compound on rivets and screws applied adhesive bond. This also counteracts the formation of crevice corrosion.
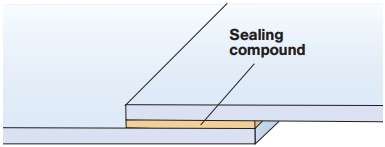 Figure 6 – Sealing crevice prevents crevice corrosion
Figure 6 – Sealing crevice prevents crevice corrosion
Source:
- How to prevent aluminium corrosion – Shapes by HYDRO
- HYDRO Design Manual – 2024