Impurities in aluminium melt
About the aluminium quality factors, such as impurities in aluminium melt.
Impurities in aluminium melt
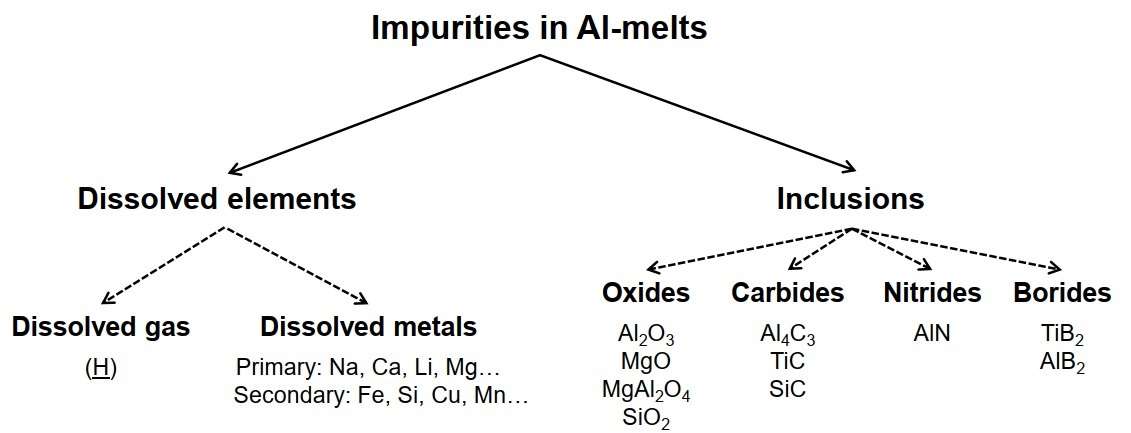
Aluminium melt is the starting material for the manufacture of any aluminium products. Different types of aluminium products require different levels of purity of aluminium melt. The quality of aluminium melt is one of the most important conditions for ensuring a given level of quality of the final aluminium products. The quality level of the melt is determined by three types of contamination [1, 2]:
- dissolved hydrogen;
- solid inclusions;
- dissolved impurity metals.
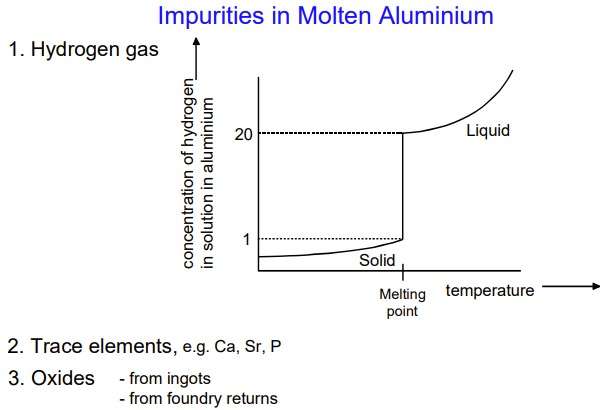 Figure 1 – Types of impurities in aluminium melt [1]
Figure 1 – Types of impurities in aluminium melt [1]
Figure 2 – Impurities in aluminium melts [2]
Contamination measurement in aluminium
The units of measurement
To assess the quality of aluminium melts, the following units of measurement are used:
- ppm = parts per million (106) = mg / kg = μg / g
- ppb = parts per billion (109) = μg / kg
- ppt = parts per trillion (1012) = ng / kg
An idea of these units is given by their analogs:
- 1 ppm = 1 a minute in two years = 1 second in 11,5 day
- 1 ppb = 1 second in 32 years
- 1 ppt = 1 second in 32000 years.
The hydrogen content
To estimate the hydrogen content, the following units are used:
- 1 ppm = 1 mg / kg = 1 μg / g
- milliliter (ml or cm3) hydrogen on 100 g of metal
- the relationship between these units: 1 ppm = 1 mg / kg = 1,12 ml / 100 g.
The content of inclusions
To assess the content of inclusions, the following units are used (Figure 3):
- ppm units, ppb и ppt;
- PoDFA scale: mm2/kg.
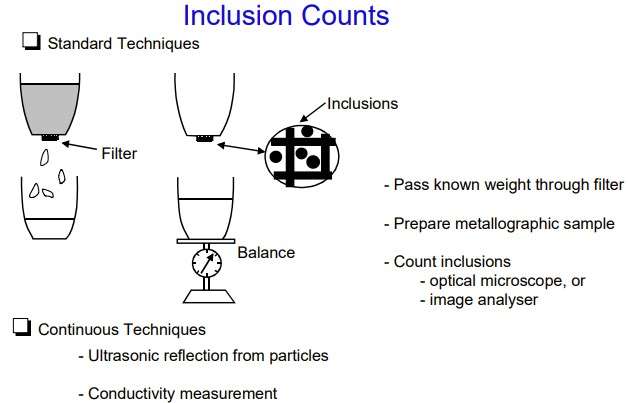 Figure 3 – The methods for counting inclusions in aluminium melt [1]
Figure 3 – The methods for counting inclusions in aluminium melt [1]
Melt quality levels of primary and recycled aluminium
The quality of aluminium melt is one of the critical issues, especially for recycled aluminium, which is obtained by remelting aluminium scrap. Typical quality levels of primary aluminium and recycled aluminium are presented in Table 1.
Table 1 – Typical contamination in primary and recycled aluminium melts [4]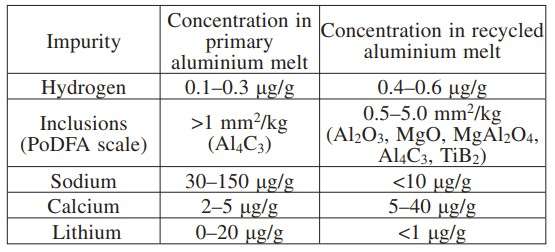
Hydrogen in aluminium
The only gas in aluminium
Hydrogen gas is the only one, which can dissolve in aluminium melt, since it does not form compounds with aluminum, unlike other gases (Figure 4 and 5):
- at 660 ºС in liquid aluminum the content of dissolved hydrogen is 0,69 ppm;
- the content of dissolved hydrogen in solid aluminium is only 0,039 ppm.
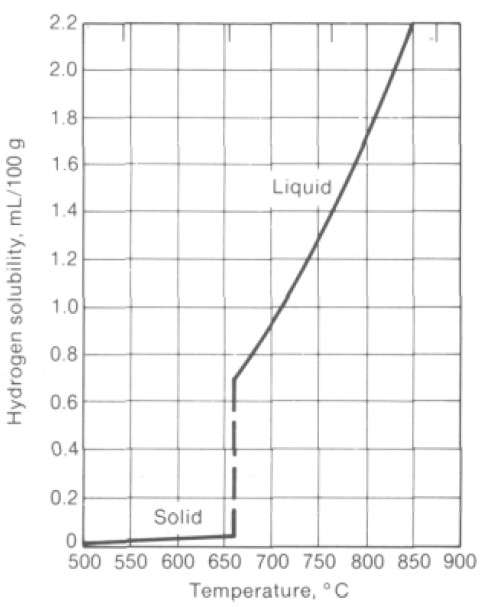 Figure 4 – Solubility of hydrogen in aluminum [5]
Figure 4 – Solubility of hydrogen in aluminum [5]
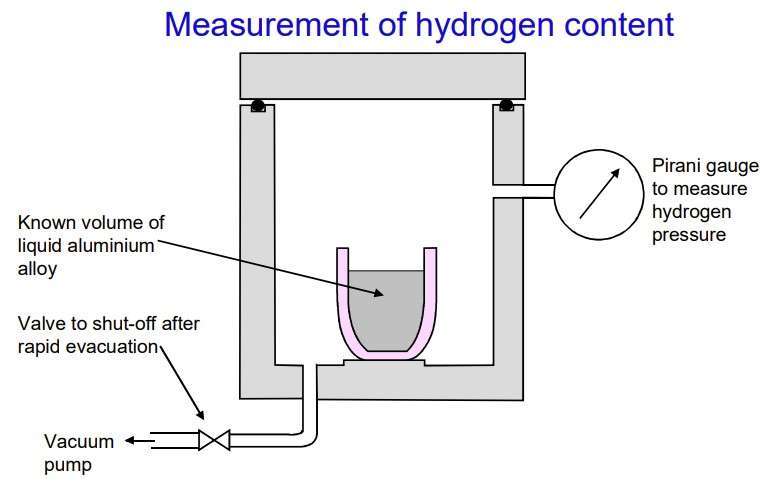 Figure 5 – The measurement of hydrogen content [7]
Figure 5 – The measurement of hydrogen content [7]
The reaction of aluminum with water
Hydrogen enters molten aluminum as a result of the reaction between the melt and water, which is in the form of moisture in the lining, on instruments, in alloying additives, fluxes and charge, as well as in the atmosphere of the furnace.
The reaction between water and aluminum is:
3H2O + 2Al → Al2O3 + 6H
As a result of this reaction, the melt is oxidized and hydrogen H2, which can disperse in the environment or enter metal. The oxidation reaction is exothermic., that is, with heat. This reaction is so chemically favorable., that almost all traces of water, which is in contact with aluminum, turn into hydrogen and oxide [2].
Oxidation of the melt surface occurs with the formation of an oxide film on it. Hydrogen is a “by-product” of aluminum oxidation. He or goes into the surrounding atmosphere in the form of molecular hydrogen H2, or is absorbed by the melt in the form of atomic hydrogen H [2].
In gas flame melting furnaces, directed to the surface of the melt, favorable conditions are created for increasing the hydrogen content in liquid aluminum, since when gas is burned, water vapor is formed:
CH4 + 2O2 → CO2 + 2H2O
Therefore, aluminum melt at temperature 700 ºС at the exit from the gas melting furnace usually has a hydrogen content 0,3-0,4 ml / 100 g [2].
Inclusions in aluminum
The influence of inclusions on the properties of aluminum products
Inclusions are unwanted solid phases., which:
- remain in the finished casting or ingot;
- reduce the level for the desired properties of the finished product.
Inclusions reduce the mechanical properties of castings and ingots, especially fatigue strength and ductility, since solid particles break down during metal forming processes or act as stress concentrators or crack nuclei during casting.
Solid inclusions can lead to:
- microscopic holes in rolled foil,
- wire breaks,
- tears in the production of beverage cans,
- point defects in thick sheets
- surface defects in sheets and extruded products.
The level of inclusions in aluminum melts for various purposes is shown in the figure. 6
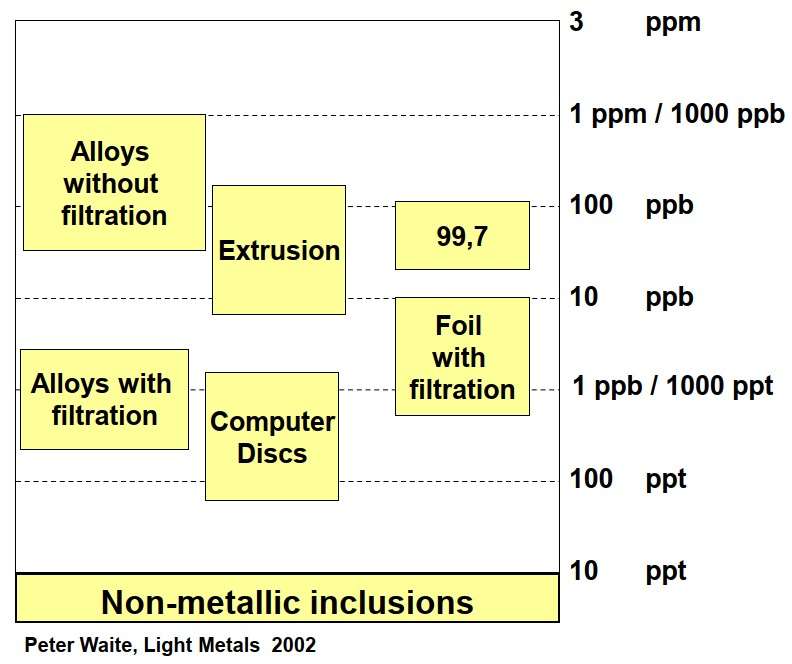 Figure 6 – Requirements for the content of inclusions
Figure 6 – Requirements for the content of inclusions
for various aluminum products [3]
Sources of inclusions in aluminum melts
Sources of inclusions are almost all technological operations of aluminum production (figure 7).
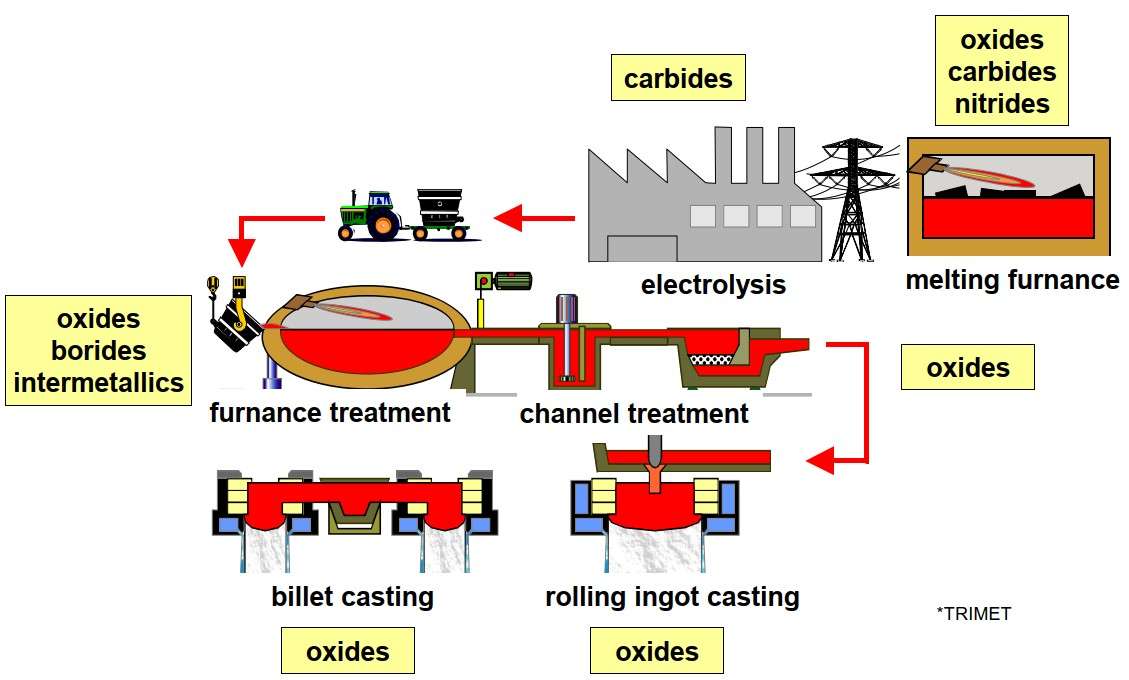 Figure 7 – Production sources of inclusions
Figure 7 – Production sources of inclusions
in aluminium melts [3]
Inclusion classification
The main problem of aluminum melts is non-metallic inclusions [3]. These include:
- oxides (Al2O3, MgO and spinel MgAl2O4) (Figure 8)
- refractory lining particles
- TiB particle clusters2 (from grinding grain)
- salts (metal chlorides)
- carbides
Examples of metallic and intermetallic inclusions are:
- Cr, CrMn и Zr (Ti) Al3
- Fe-Si
- incompletely dissolved alloying elements
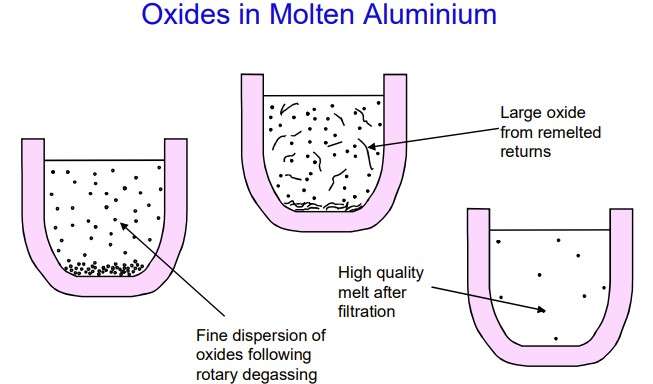 Figure 8 – Oxides in molten aluminium [1]
Figure 8 – Oxides in molten aluminium [1]
Impurity metals
The main sources of impurity metals in aluminum are [1, 2]:
- raw materials for primary aluminum, such as alumina and coke;
- scrap aluminum products, contaminated with various “non-aluminum” components;
- technological processes;
- production technological equipment.
In the primary aluminum melt:
- Sodium is present, lithium and calcium, that get there from the electrolyte
- The impurity metals with the highest concentration are iron and silicon from alumina
- In small concentrations, primary titanium is also usually present in primary aluminum., vanadium, manganese, copper, magnesium,, brown.
In the secondary aluminum melt:
- Iron, copper and zinc are the main metal pollutants;
- Lead, chromium, lead, nickel may be contained in small quantities. For most wrought alloys, the limit for them is 0,05 %.
- Melt from mixed aluminum scrap may only be suitable for the production of secondary casting alloys. The production of secondary deformable aluminum alloys requires careful sorting of aluminum scrap and removal of foreign metals.
Sources:
1. The Liquid Metal – TALAT Lecture 3202 /John Campbell and Richard A. Harding – 1994
2. Inclusions and Hydrogen and Their Effects on the Quality of Direct Chill Cast and Flat Rolled Aluminium Alloys for Aerospace Applications /A. J. Gerrard – PhD thesis – The University of Birmingham – 2014
3. Understanding of Inclusions – Characterization, Interactions and Boundaries of Removability with Special Focus on Aluminium melts / Bernd Friedrich – Aachen University – 2015
4. V. Kevorkijan – Materials and technology 47 (2013) 1, 13-23
5. Aluminum and Aluminum Alloys / ed. J.R. Davis – ASM International – 1993