Aluminium melting fluxes
Improve the quality of aluminium through fluxing. Discover how aluminium melting fluxes can degas, reduce magnesium, remove impurities, and enhance the alloying process.
What is flux?
Term
The term “fluxing” used to describe all the aluminium melt treatments, in which special chemical compounds are used. They are called “fluxes” [1]. These compounds are generally inorganic and can perform several functions, such as , degassing, magnesium reduction, removal of impurities and alloying. “Fluxing” also includes the treatment of the melt with inert or reactive gases to remove inclusions or gaseous contaminants from the metal [1].
Chemical composition of fluxes
Fluxes in solid state, as a powder, flakes or granules, usually consist of chlorides and fluorides with additional additives to impart special properties.
- Most fluxes for melting aluminium based on a mixture of KCl and NaCl salts, which form a low-temperature (665 ºС) eutectic.
- Another common ingredient of the flux for melting aluminium is sodium fluoride NaF, which forms a ternary eutectic with KCl and NaCl at 607 oC.
- A normal coating flux contains about 47,5 % NaCl, 47,5 % KCl and 5 % a fluoride salt.
- Low melting temperature is an important, so it increases the fluidity of flux.
The role of fluoride in fluxes
- Fluoride salts of alkali metals act as surfactants, lowering the surface tension between the flux and metal, one side, and flux and oxides, with another.
- Chloride salts exhibit this property to a lesser extent [1].
- Alkali metal fluoride salts have the ability to dissolve oxides, which facilitates their penetration into the oxide films in the slag and growths on the walls of the melting furnace.
- This leads to improved wettability, which promotes the separation of oxide inclusions from the melt and the aluminum metal from the dross.
Exothermic fluxes
Nitrate additives, such as KNO3, leads to the release of heat. These fluxes are exothermic. Oxigen is liberated from the decomposition of the nitrate and reacts with the metallic aluminium to form Al2O3 with a significant amount of heat. This reaction increases the penetration of flux into the furnace wall.
Degassing fluxes
Certain compounds decompose with evolution of gases, for example, chlorine or carbon dioxide. If such fluxes enter under the melt surface, they form bubbles, which reduce the hydrogen content in melt. The best known of degassing fluxes for melting aluminium is hexachloroethane (C2Cl6), which allocates chlorine Cl2 and a gaseous compound of AlCl3.
Hydrogen and oxides in aluminium melt
The reaction of aluminum with water
Hydrogen and oxides are common impurities in aluminium melt. Their source is water from the atmosphere. The reaction between aluminum melt and water is shown in Figure 1. In this case the aluminas can be crystallized in a very hard corundum crystal structure, shown in Figure 2. This can happen, for example, on the wall of the melting furnace.
 Figure 1 – The reaction of formation of oxides and hydrogen in aluminium melt [2]
Figure 1 – The reaction of formation of oxides and hydrogen in aluminium melt [2]
How rise the hydrogen bubbles
When aluminium solidifies, the solubility of atomic hydrogen in it drops sharply (Figure 2). As a result, hydrogen atoms in the molecule and are combined in the hardened metal bubbles occur. The Figure 4 schematically illustrates the formation of oxides, and the occurrence of hydrogen bubbles.
 Figure 2 – Solubility of hydrogen in aluminium [2]
Figure 2 – Solubility of hydrogen in aluminium [2]
Atmospheric moisture reacts with molten aluminium to form an oxide film and the atomic hydrogen in between the aluminium atoms. Oxides do not remain only on the surface, some of them also fall into the melt and, when the temperature is reduced, some of these oxides having hydrogen bubbles (Figure 3).
 Figure 3 – Formation of a hydrogen bubble around the oxide film [2]
Figure 3 – Formation of a hydrogen bubble around the oxide film [2]
How fluorides purify aluminium melt
The oxides can be removed from molten aluminum by flux processing of melt. Fluxes based on fluorides able to bind oxides. The interfacial tension between the metal oxide and considerably higher, than the interfacial tension, which occurs between oxide and fluoride. Then, fluorides and oxides form mixed phases, because as a result of their lower energy state fluorides “stick” to oxides and cover them, and aluminum is separated from these mixed phases [2, 3].
Aluminium in dross
Dross, which is formed in the melt during processing without the use of flux:
- Coomonly dross has a high aluminum content, usually 80 to 95 %.
- After melt flux processing, dross is less dense and has an aluminum content of 15 to 35 % (Figure 4) [2, 3].
 b
b
Figure 4 – Aluminium dross [2]:
a – high aluminium content; b – low aluminium content
Cleaning melter walls of oxides
Cleaning the walls of the furnace from corundum is as follows [2]. Before cleaning, the oven must be nearly empty and heated to a high temperature between 800 и 900 oC. The burners in the furnace is turned off. The flux for cleaning the furnace is sprayed on areas with corundum build-ups and around them (Figure 15). The furnace is closed on 30-40 minutes. The furnace was then opened and the purge walls conventional tool. Thus corundum must be easily separated from the walls of the furnace.
Figure 5 – The operation of applying flux to the walls of the furnace [2]
Exothermic fluxes and fluxes based on fluorides
There is a special group of exothermic flux based on nitrates. Between aluminum and nitrates exothermic reaction occurs with a local increase in temperature to 2000 oC. Under the influence of this high temperature aluminum viscosity becomes very low, and therefore it can easily flow from the dross. The disadvantage here is that, that the low metal content in the slag is achieved due to the additional formation of oxides in the melt [3].
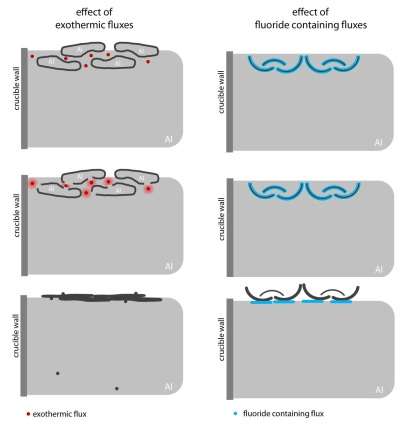 Figure 6 – Differences in the action of exothermic fluxes and fluxes with fluoride salts [3]
Figure 6 – Differences in the action of exothermic fluxes and fluxes with fluoride salts [3]
Sources:
- The Properties and Uses of Fluxes in Molten Aluminium Processing / T.A. Utigard et al – JOM, November, 1998
- Mechanisms in the Cleaning of Aluminium Melts with Flux Preparations – Presentation – SCHÄFER Metallurgie GmbH

