How the crystal structure of aluminium provides its properties
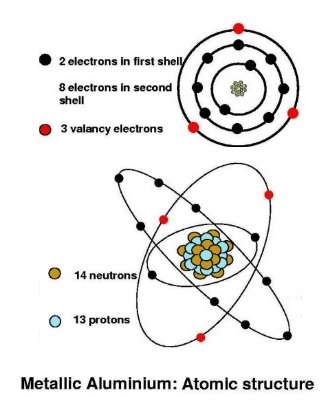
The atomic number of an aluminium atom is 13. The outer electron shell contains three electrons. These contribute to the “free electron gas” of aluminium crystals and give such crystals excellent electrical conductivity. As a general rule, the electrical conductivity is reduced by the addition to aluminium of impurities and by the deliberate addition of other elements to form aluminium alloys.
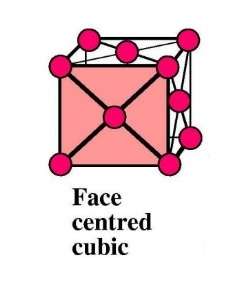
Aluminium atoms assemble into an array to form a crystal lattice. The crystal lattice has a face-centred cubic structure (often abbreviated to fcc). Aluminium, in common with most other metals and their alloys, pack atoms together in a highly compacted way.
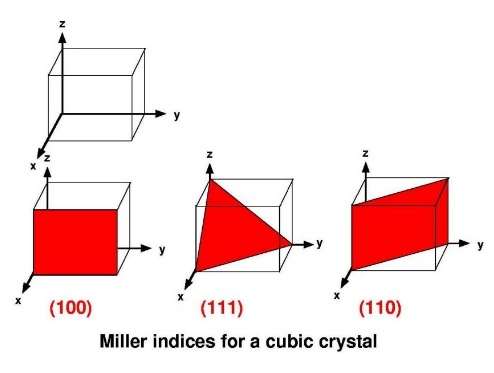
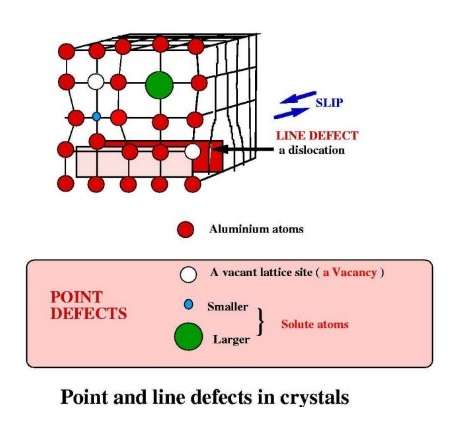
The slip of one raft of atoms over a neighbouring raft is the mechanism by which a crystal can change its shape. That is, by the application of a sufficient force to a crystal, it responds by changing its shape. This is known as plastic deformation. The plane on which the slip occurs is determined by the geometry of the crystal structure.
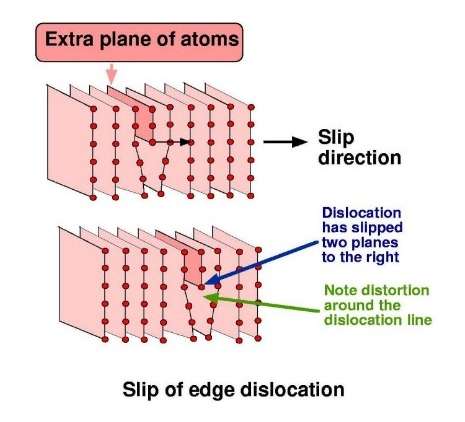
Deformation by slip in aluminium mostly occurs on (1, 1, 1)-planes and along directions (1, 1, 0). In practice, the energy that would be required to move a complete raft of closepacked atoms all in one go would be impossibly large, and this never happens. Instead, slip occurs by the movement of so-called line defects in the crystal, known as dislocations. The dislocation line is the boundary of an extra plane of atoms. Slip occurs by the movement of this extra plane along the slip plane; hence, deformation is a localised process and the energy required is very much less.
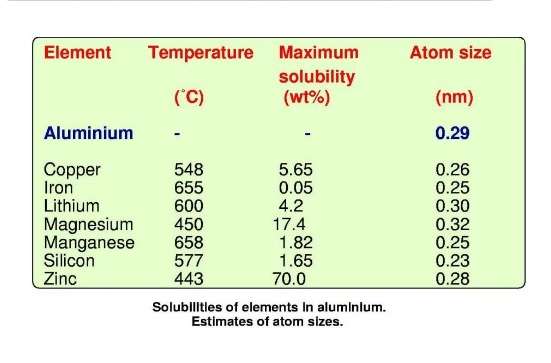
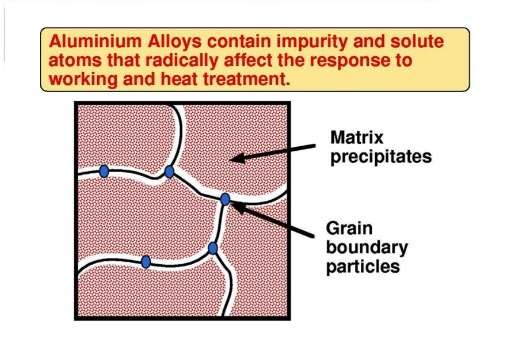
In the real world, very high purity aluminium is a rarity. Normally aluminium material contains at least a small amount of impurities and, for most engineering applications, the material contains deliberate additions of alloying elements. The main impurities in commercially-pure aluminium are iron and silicon. Iron has a very low solubility in aluminium (0,05 wt % maximum at 665 °C). Hence an impurity level higher than this results in particles of aluminium-iron intermetallic which are insoluble. Some elements, notably copper, lithium, magnesium, manganese, silicon and zinc dissolve, each up to a certain limit in the aluminium lattice. They form so-called solid solutions.
In a solid solution, the ‘foreign’ atom substitutes for an aluminium atom. These foreign atoms are known as solute atoms. They have atom sizes that are different from the atom size of aluminium. This means that solute atoms introduce a distortion into the aluminium lattice. It is a feature that has a controlling influence on how the alloy responds to heat treatment and to mechanical working.
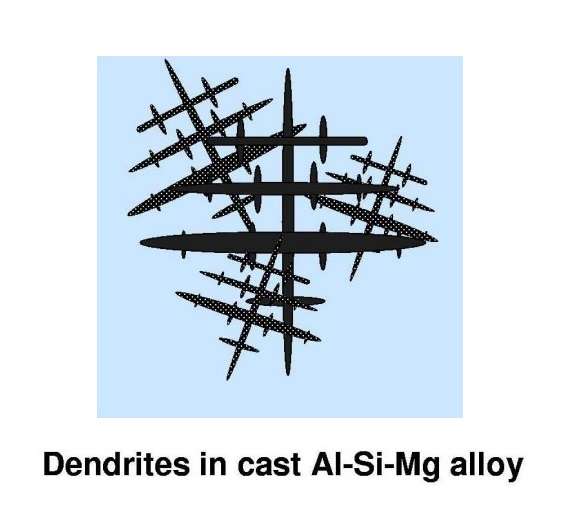
In most situations where aluminium is processed and applied as an engineering material, the bulk material is composed of an agglomeration of a large number of crystals, so-called grains. So next, we must look at the formation and characteristics of multi-grained materials. As the liquid aluminium cools to, and then below, the freezing point, small crystals of solid start to grow within the liquid. The solid crystal grows at different rates in different lattice directions, and the crystal takes on a characteristic, so-called ‘dendritic’ morphology. The sizes of the dendrites are controlled by the conditions of freezing, that is, by the method by which the bulk material is cast.
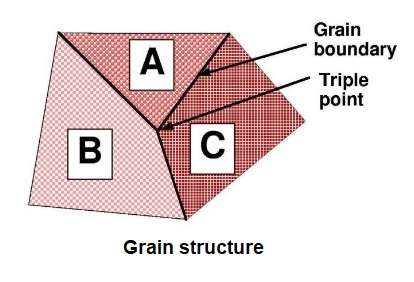
Fully solid cast material consists of colonies of dendrites, separated by grain boundaries. The boundaries between grains are in a state of local, high disorder and are of higher energy relative to the regular lattice of grain interiors. Thermodynamic principles say that systems always tend to minimise their total energy; consequently, a sustained annealing treatment leads to growth of grains to lower the total grain boundary area. This same principle is the main driver that determines how a material responds to deformation (working) and further heat treatments.
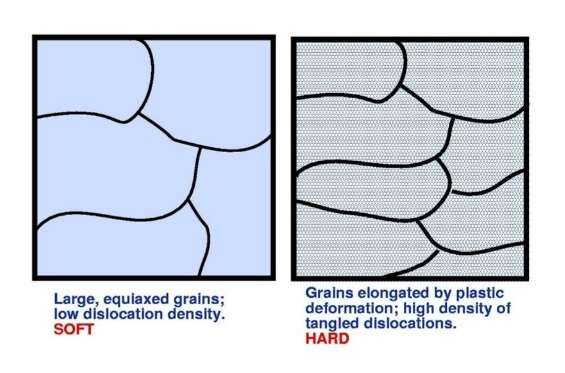
Let’s look at a slab of commercially pure aluminium that has been fully annealed and homogenised. The aluminium grains are reasonably uniform in size (equiaxed) and of low dislocation density. Hence, the material is soft and easily deformed. If the material is mechanically worked at room temperature, it hardens noticeably. This is called work hardening. The plastic deformation creates a multitude of dislocations, whose ability to slip becomes progressively more difficult because of resistance from neighbouring dislocations. Macroscopically the material becomes ‘hard’.
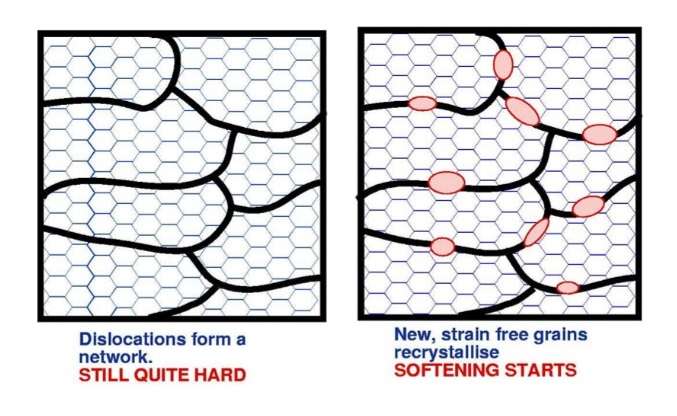
If the temperature of the slab is now raised to around 250°C, the dislocations will form a network structure of cells (polygonisation). The process is called recovery and the material will start to soften slightly.
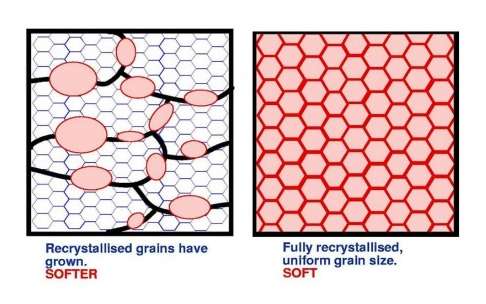
If the temperature of the slab is raised further, new strain free crystals are nucleated; recrystallisation has commenced and the material starts to soften. This process continues until the whole of the material has recrystallised and softened.
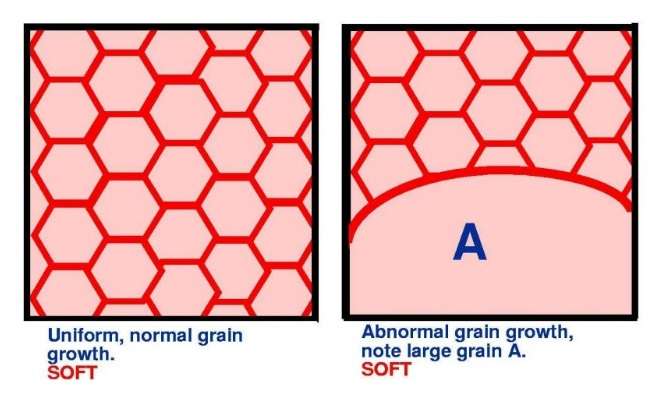
If a fully recrystallised material is held at elevated temperature for a period of time, then the grains grow. Initially the growth is reasonably uniform but later, a small number of grains grow preferentially and abnormal grain growth sets in.
In contrast to pure aluminium, aluminium alloys contain solute additions which can markedly effect grain structures and particularly the microstructures within the grains. This in turn strongly influences the responses of alloys to working and heat treatment. Both crystal structure and microstructure influence mechanical properties. Slip is inhibited by grain boundaries, which are disordered regions. Slip can also be made difficult by dispersing particles of another phase throughout the matrix.
The source:
TALAT Lecture 1201 – Introduction to Aluminium as an Engineering Material / M H Jacobs – European Aluminium Association – 1999