Aluminium melting: a dry hearth versus a wet bath
The purpose of an aluminium melting furnace is to convert the metal from its solid phase to liquid as quickly and efficiently as possible. The industry has many versions of the melting furnace, each with its own benefits and limitations. The simplest and probably most widely used are the single-chamber melting furnaces:
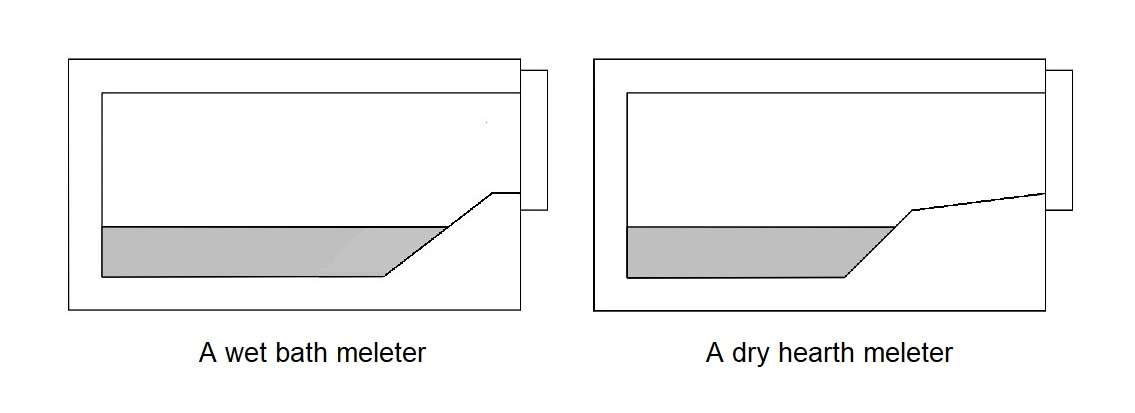
- A gas-fired reveberatory furnace with metal charge directly into bath (a wet bath melter)
- A gas-fired reverberatory furnace with metal charge on dry hearth (a dry hearth melter).
The efficiency of these furnaces has its limitations, which are related to the physical properties of aluminum, solid and liquid:
- Solid aluminium is approximately 15 percent more dense than molten aluminium. Therefore it will sink under the surface of the bath, significantly reducing heat transfer.
- When melting pure aluminum from solid metal at room temperature to liquid metal at 750 degrees Celsius from 85 to 90 pecent of the energy is being transferred to the metal whilst it is in a solid form, potentially submerged beneath the molten bath.
- Liquid aluminium has a thermal conductivity about 50 percent lower than solid aluminium. This limits energy movement from the surface of the bath down to the solid charge material.
- In a typical furnace, metal temperature variation from the top to the bottom of the static bath is from 60 to 70 degrees Celsius. Any solid material resting on the floor of the furnace will therefore melt at a much slower rate.
- A dross layer reduces the heat transfer coefficient of the bath surface, further limiting the energy available for melting submerged solid materials.
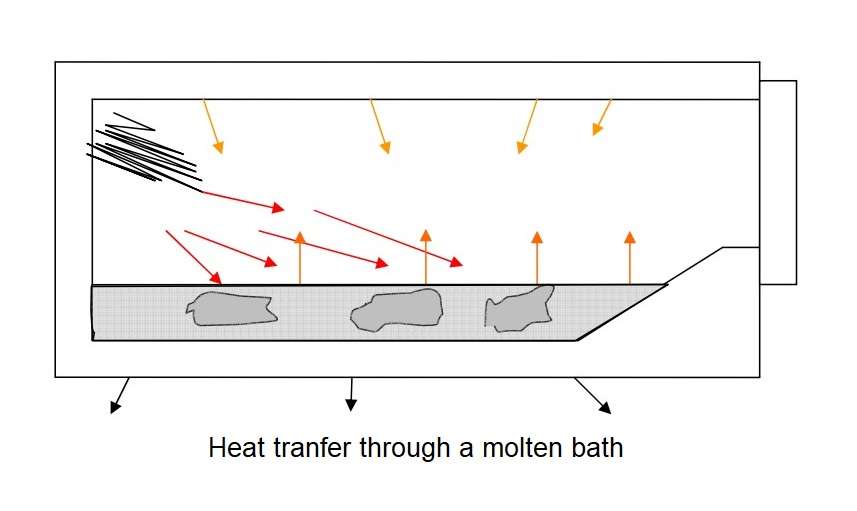
Now first about a wet bath melter. Solid aluminium charge is placed directly into the furnace melting area. In this case the charge material must be completely dry and free of liquid contaminants. The advantages of this furnace solution are simplicity, low cost and versatility. The figure shows solid aluminium submerged beneath the surface of the molten bath. Once submerged, heat transfer to the solid metal can only be indirectly through the surface of the liquid bath. Heat transfer to the bath heat is by a combination of radiation from the refractory surfaces and from the burner flame envelope, as well as forced convection from the products of combustion that circulate through the chamber.
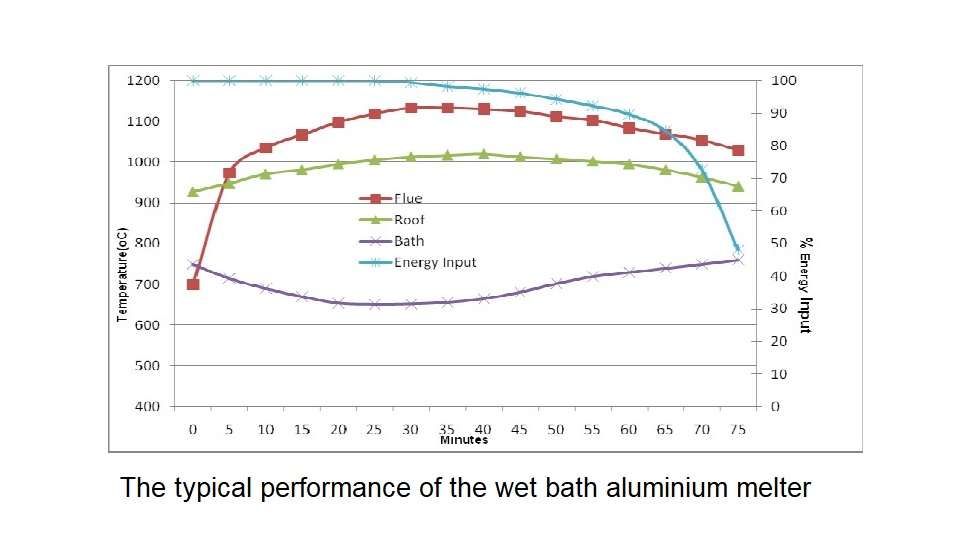
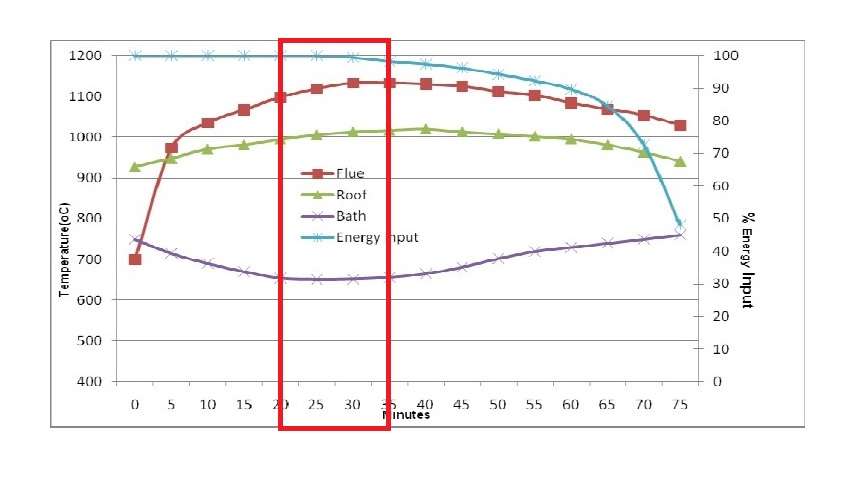
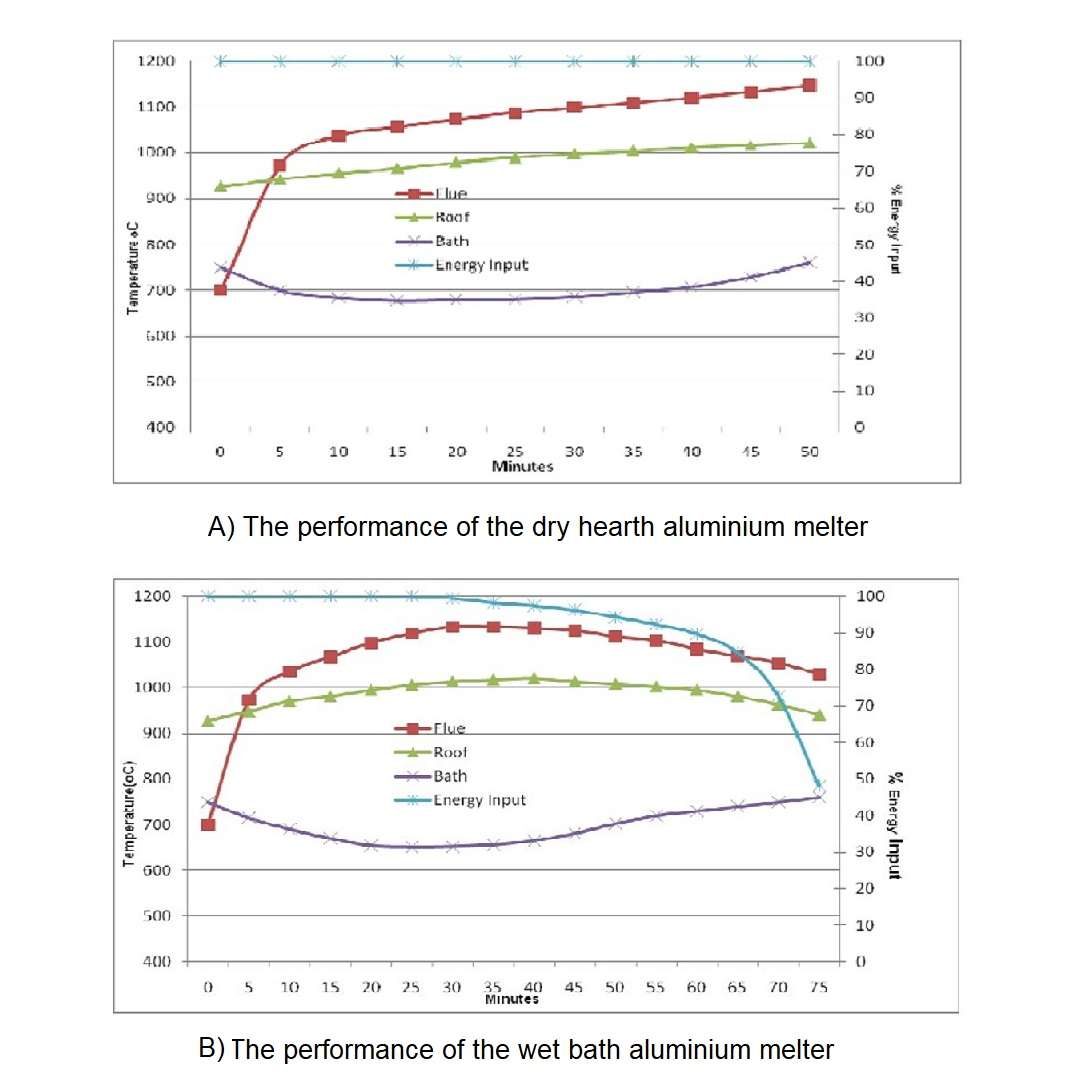
Figure 3 shows the typical performance of the wet bath aluminium melter where 4000 kilograms of ingots or scrap with minimal preheat have been charged directly into a molten bath of above 10000 kilograms. Four stages can be defined from the operation chart. They help to understand how aluminium melts.
Stage 1: from 0 to 20 minutes: Initial thermal input to the furnace is very high to recover heat lost from the door opening and because some solid material will remain exposed above the metal line. Bath temperature drops rapidly as the charge material cools the molten metal to a temperature that is close to solidification. Stored latent heat of the molten metal is released as the cooling occurs, and this tends to prevent the bath temperature from dropping below 650 degrees Celsius.
Stage 2: from 20 to 35 minutes. Flue temperature and roof temperature rapidly rise to the set point. The bath temperature is maintained at approximately 650 degrees Celsius due to the latent heat of melting aluminium and the phase change continues to absorb energy from the bath. Thermal input must reduce because the refractory surfaces have reached the operational limit. Heat transfer at this stage is primarily through the surface of the molten metal.
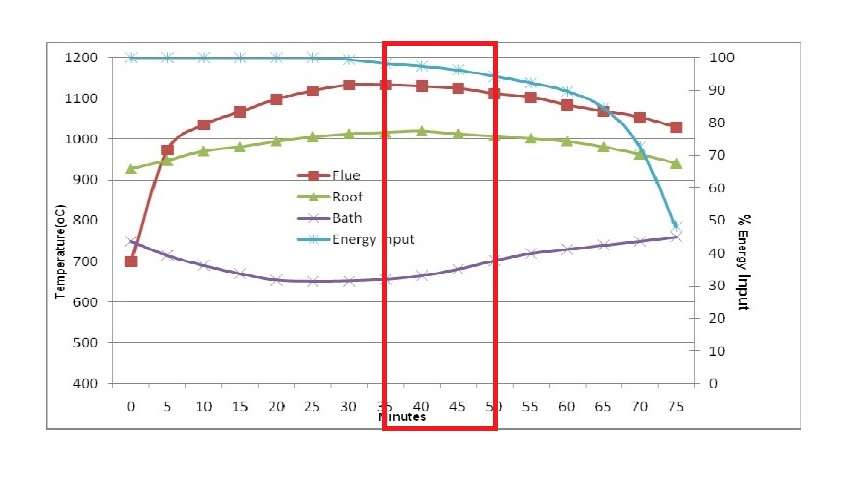
Stage 3: from 35 to 50 minutes. Bath temperature increases because the solid metal within the furnace has reduced. Rate of energy transfer through the surface of the bath now exceeds the cooling effect of melting aluminium and casing heat loss.
Stage 4: from 50 to 75 minutes. Thermal balance must be maintained and so the burner system output is reduced accordingly. This last part of the melting cycle delivers the final from 7 to 10 percent of the total energy to the metal but requires more than 30 percent of the cycle time. Heat transfer in this final stage is constrained by the surface coefficient of the molten bath.
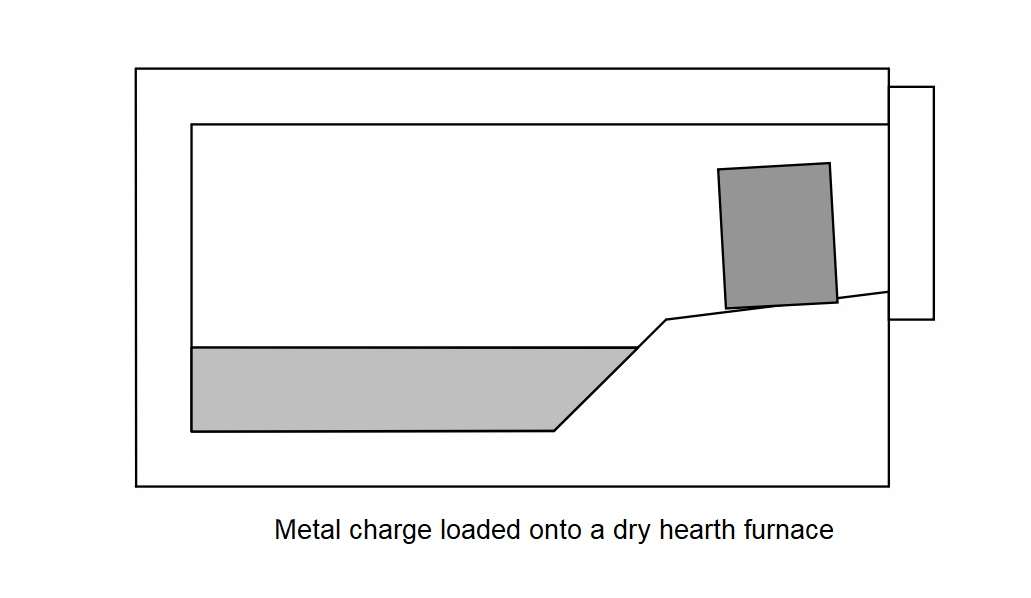
Now about a dry hearth melting furnace. This furnace have an extended and elevated charge ramp or sill enables the solid metal to either be preheated or partially melted without coming into contact with the liquid bath. Metal placed on the charge hearth rapidly absorbs energy from the furnace by radiation and convection. Metal remains on the dry hearth until the bath temperature has recovered back to set point. At this time the preheated and semi-molten charge is pushed into the bath and cold metal is placed onto the dry hearth.
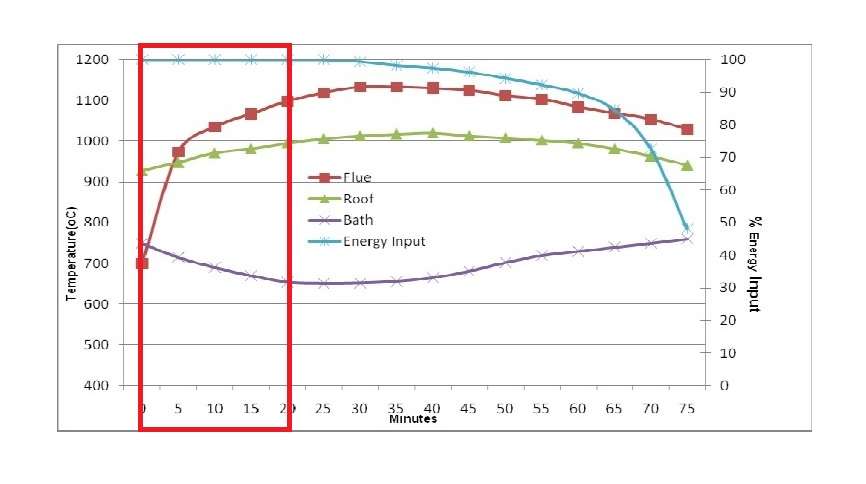
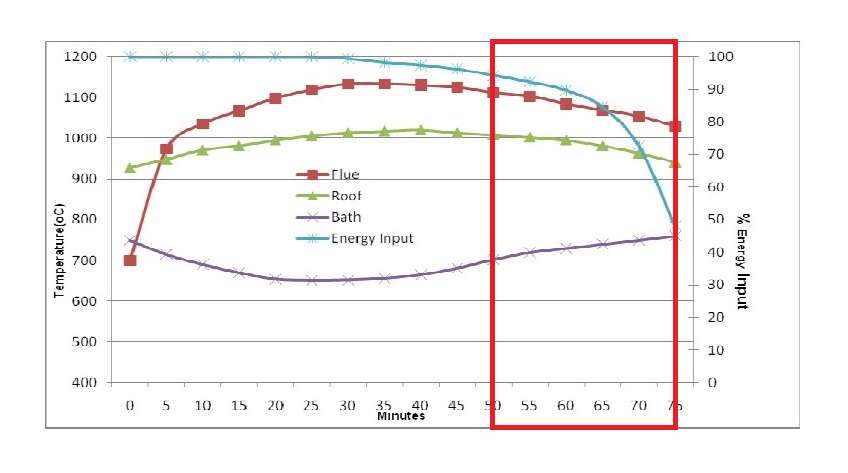
The figure 9A shows the typical performance of the dry hearth aluminium melter where 4000 kilograms of ingots or scrap have been charged into a dry hearth of the 16 tonne holding capacity furnace with a molten bath of above 10000 kilograms.
In comparison with the wet bath melting furnace shown in figure B the melting technique is far superior to charging cold metal into a wet bath. From the figures A and B it can be established that:
- Average melting rate improve by 25 to 30 percent.
- Energy consumption reduced by 10 to 15 percent.
- Furnace utilisation is at maximum.
The principal reason for the improvement is that approximately from 50 to 70 percent of the energy is being transferred to the ingots before they are submerged into the molten bath. The total energy required to be absorbed through the bath surface has now been reduced by around 50 percent.
And in conclusion. The concept of melting aluminium on a dry hearth offers following benefits:
- Charge material is directly exposed to high velocity products of combustion and furnace chamber radiation throughout the melting cycle.
- As the solid charge is never submerged under molten metal, the melting rate per unit of area of hearth is much higher than for a wet hearth furnace.
- Solid material can be completely separated from the molten bath, virtually eliminating the risk
of a steam explosion caused by wet charge material.
The sources:
- Dry Hearth Melting Furnaces /Peter Newman // Aluminium Cast House Technology XI – 2010
- Reverberatory and Stack Furnaces / D. White, M. Reeves, P. Campbell and D. Neff // ASM Handbook, Volume 15: Casting – 2008